Doc Robinson
Forum Replies Created
-
AuthorPosts
-
Doc Robinson
ParticipantI just learned about the existence of these recent songs by Van Morrison and Eric Clapton:
No More Lockdown by Van Morrison
https://youtu.be/uUeuhM-NSjUStand and Deliver, written by Van Morrison and performed by Eric Clapton
https://youtu.be/d0cCJMrNZa8
Stand and deliver
You’ve let them put the fear on you…Doc Robinson
ParticipantAn article in Science today about the risks of anaphylactic reactions to the Polyethylene Glycol (PEG) in the Pfizer and Moderna vaccines:
Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactionsSevere allergy-like reactions in at least eight people who received the COVID-19 vaccine produced by Pfizer and BioNTech over the past 2 weeks may be due to a compound in the packaging of the messenger RNA (mRNA) that forms the vaccine’s main ingredient, scientists say. A similar mRNA vaccine developed by Moderna, which was authorized for emergency use in the United States on Friday, also contains the compound, polyethylene glycol (PEG).
PEG has never been used before in an approved vaccine...
PEGs were long thought to be biologically inert, but a growing body of evidence suggests they are not. As much as 72% of people have at least some antibodies against PEGs, according to a 2016 study led by Samuel Lai, a pharmaco-engineer at the University of North Carolina, Chapel Hill, presumably as a result of exposure to cosmetics and pharmaceuticals. About 7% have a level that may be high enough to predispose them to anaphylactic reactions, he found…
Nevertheless, the companies were aware of the risk. In a stock market prospectus filed on 6 December 2018, Moderna acknowledged the possibility of “reactions to the PEG from some lipids or PEG otherwise associated with the LNP.” And in a September paper, BioNTech researchers proposed an alternative to PEG for therapeutic mRNA delivery, noting: “The PEGylation of nanoparticles can also have substantial disadvantages concerning activity and safety.’”
He notes that both mRNA vaccines require two shots, and he worries anti-PEG antibodies triggered by the first shot could increase the risk of an allergic reaction to the second or to PEGylated drugs…
Doc Robinson
ParticipantVietnamVet: “CDC report of 3150 health impact events in 112,807 injections (2.7%)”
From the CDC site:
as of Dec 18,
112,807 registrants with recorded first dose,
with 3,150 health impact events (unable to perform normal daily activities, unable to work, required care from doctor or health care professional.)That’s about 3% of those who got the first shot.
This percentage has been increasing every day since the shots began.
The second shot is supposedly going to be worse.From page 6 of this CDC presentation:
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-CLARK.pdfDoc Robinson
ParticipantFour days ago, the International Air Transport Association revealed how their IATA Travel Pass would work as a digital passport, for travelers to “securely manage their travel in line with any government requirements for COVID-19 testing or vaccine information.”
It involves downloading a free app to your smartphone, taking a selfie, then proving that you are alive, then uploading biometric data from your passport chip…
The process that a passenger would take to securely identify themselves in the IATA Travel Pass uses government issued ePassports to create a digital travel credential as per the standards developed through ICAO.
The process has six steps:1. Download the free IATA Travel Pass to their Smart phone and login
2. Take a selfie with the smart phone
3. Complete a liveness test as instructed by the phone – i.e., move their head, close their eyes in front of the camera as instructed
4. Scan the data on the two lines at the bottom of the passport photo page with their smart phones and scan the data-chip on the passport as prompted by the phone
5. The IATA Travel Pass then matches the photo with the passport data (which contains a digital biometric photo of the passport holder) to verify that (1) the passport belongs to the person in front of the phone and (2) that the passport is genuine and has not been tampered with.
6. The verified digital travel credential is then stored on the passenger’s phone and can be used as their ‘digital passport/ ID’.Doc Robinson
Participant@ teri
Despite its potential for causing anaphylactic reactions in people, Polyethylene glycol (PEG) has a functional role in the mRNA vaccines, as described in this article from 2016:.
mRNA vaccine delivery using lipid nanoparticles [LNPs]
The PEG coating strongly influences the properties of the LNPs and has to be tailored carefully. A higher PEG content usually increases the blood circulation time of LNPs, while reducing cellular uptake and interaction with the endosomal membrane…The right amount of PEG coating on the LNPs is critical… However, anti-PEG antibody response following repeated intravenous (IV) administration of PEGylated LNPs has been reported to dramatically accelerate blood clearance of the LNPs and to lead to acute hypersensitivity. This finding is very concerning for immunotherapy applications, where multiple dosing may be required for long-lasting protection.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5439223/
Regarding polyethylene glycol (PEG) found in various products, a 2019 medical journal article says “…mild to life-threatening immediate-type hypersensitivity reactions have been reported, with clinical manifestations ranging from generalized urticaria to anaphylactic shock.”
But, “no studies have examined the prevalence of type 1 PEGs hypersensitivity, so its incidence may have been underestimated.”
https://aacijournal.biomedcentral.com/articles/10.1186/s13223-019-0327-4
Doc Robinson
ParticipantFrom the Journal of the American Medical Association (JAMA):
Behaviorally Informed Strategies for a National COVID-19 Vaccine Promotion ProgramThe US needs a national strategy for promotion of COVID-19 vaccines that unites the urgency and commitment of Operation Warp Speed with innovative behavioral science and social marketing approaches to increase COVID-19 vaccine confidence and acceptance in diverse populations…
Make Access to Valued Settings Conditional on Getting Vaccinated. Many retail outlets and restaurants have made face coverings mandatory for the protection of employees and other customers. In a similar way, access to certain settings could be made conditional on receiving the COVID-19 vaccine: health care clinical settings (with the exception of individuals presenting with acute illness or emergencies); congregate living facilities (nursing homes, college dormitories); kindergarten to 12th grade schools; workplaces, particularly for large employers and settings where employees interact with the public (retail, grocery); and public institutions. Individuals could also be required to show proof of vaccination to enter stores, movies, in-restaurant dining, amusement parks, gyms, bars, and other public places with substantial risk of transmission. Qantas Airlines, for example, reports that it and other airlines are considering making vaccination a condition for international air travel.
These approaches could provide a strong incentive to get vaccinated. The degree of acceptability of these approaches will depend on the public’s perceptions about COVID-19 by the time vaccines are widely available; a long winter of new cases, overcrowded hospitals, and more than 2000 COVID-19 deaths per day may shift perceptions of what is politically acceptable. Employers can legally mandate vaccination as a condition for in-person work provided there are exceptions for concerns related to disabilities and religious beliefs and reasonable alternatives to continue to work for those who refuse to vaccinate, such as working from home.
Doc Robinson
ParticipantIt’s official.
“Federal agency says employers can require workers to get COVID-19 vaccine“
Doc Robinson
ParticipantFDA investigating allergic reactions to Pfizer vaccine reported in multiple states
Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research… said the FDA was not certain what caused the reactions but indicated a chemical called polyethylene glycol, which is present in the vaccines produced by Pfizer and BioNTech as well as by Moderna “could be the culprit.” He added that the reaction some people have experienced could be more common than once thought.
Doc Robinson
ParticipantFrom an interesting editorial published in The Lancet yesterday:
“…the world has never before needed to implement mass immunisation of its entire adult population..”COVID-19 vaccines: the pandemic will not end overnight
…this first step in what will need to be a global mass immunisation programme will not immediately end the COVID-19 pandemic. Although control over the infection’s most harmful effects is expected and limiting its spread can be hoped for, it will likely be a few years before the virus can be brought under control worldwide…
Even for the vaccines for which data are publicly available, many unknowns with a bearing on the effectiveness of immunisation programmes remain. How long does immunity last?… Do any of the vaccines prevent viral transmission?… Are the vaccines safe and efficacious in populations that have not been included in trials and might be at increased risk of severe disesase, such as pregnant women?… The unknowns of how the vaccine affects transmission makes the possibility of achieving herd immunity through vaccination uncertain.
In addition to these unknowns, the world has never before needed to implement mass immunisation of its entire adult population. The challenges it entails range from financial, to logistic, to social…
https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(20)30226-3/fulltext
Doc Robinson
ParticipantFor the Pfizer vaccine, the CDC seems to be throwing caution to the wind, by giving a green light for these people to be vaccinated:
Immunocompromised
Pregnant
Lactating
History of any allergies or anaphylaxis not related to vaccines or injectable therapiesA yellow light is given for people with a severe acute illness, or with a history of anaphylaxis resulting from another vaccine. These people are recommended to get a risk assessment and maybe (just maybe) skip the vaccine for now.
The only red light (or contraindication) is given for people with a history of severe reaction (e.g., anaphylaxis) to any component of the Pfizer vaccine. “Do not vaccinate” these people.
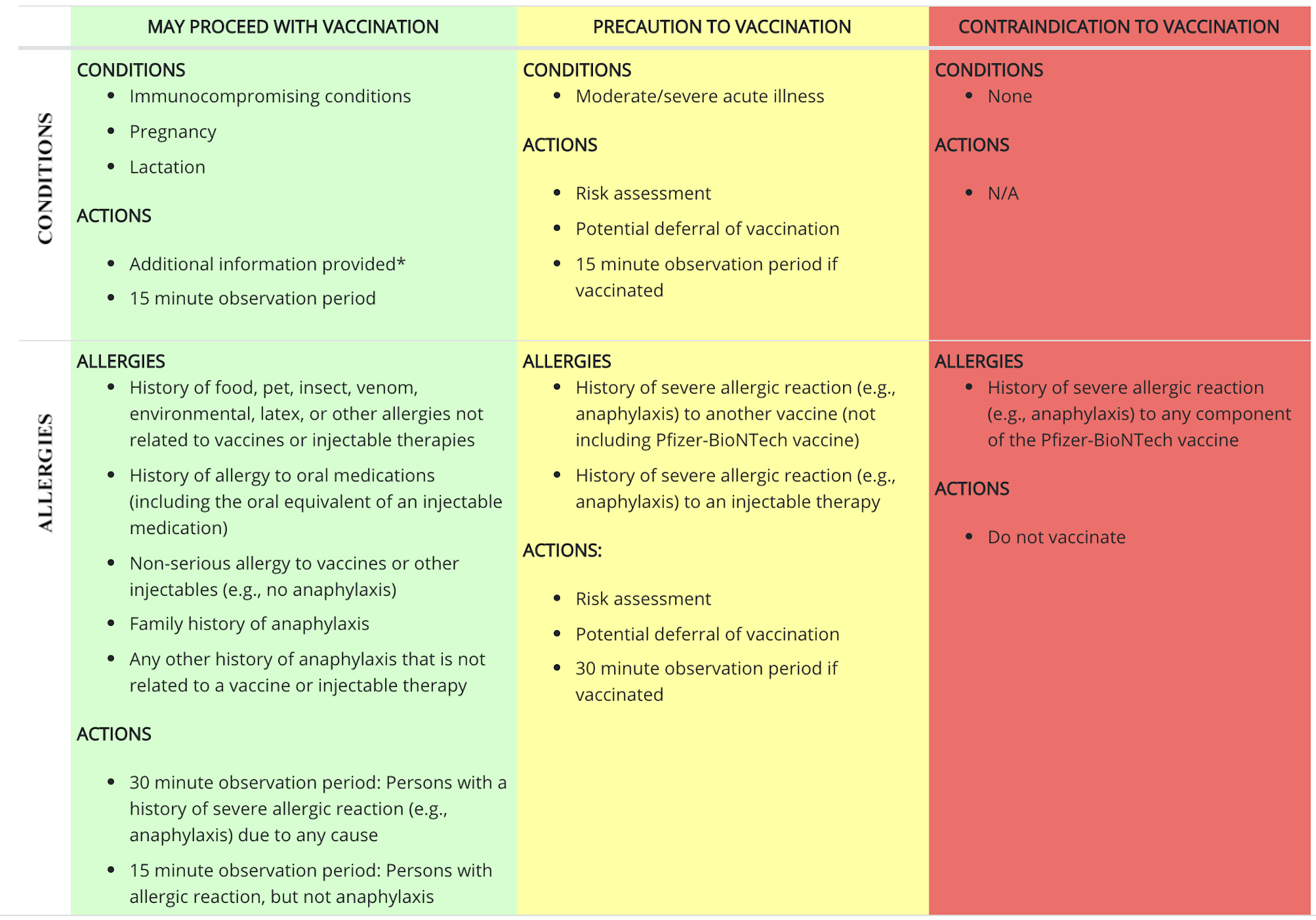
Clinical guidelines from the Centers for Disease Control and Prevention for determining which patients should receive the Pfizer-BioNTech COVID-19 vaccination.https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/clinical-considerations.html
Doc Robinson
ParticipantPentagon Training Equates Whistleblower Chelsea Manning With Terrorists
“…Another slide in the presentation lists Manning’s alleged “anti-American statements” as a “pre-attack indicator.”What’s next, preemptive arrest and jailing to prevent the “terrorists” from blowing the whistle?
Doc Robinson
ParticipantToday the advisory committee voted in favor of the Moderna vaccine, as expected. Although one expert didn’t vote yes, for strong reasons. “The question: Based on the totality of scientific evidence available, do the benefits of the Moderna Covid-19 vaccine outweigh its risks for use in individuals 18 years of age and older? The panel’s answer: 20 votes in favor and one abstention.”
…Why the abstention?Michael Kurilla, the director of clinical innovation at the National Center for Advancing Translational Sciences, part of the NIH, said that he was uncomfortable that wording of the question was too broad and too positive for an emergency use authorization, a protest he had registered before the vote was made. He said afterward that he would have preferred to target the regulatory clearance to people at high risk. “A blanket statement for individuals 18 years and older is far too broad,” he said.
He also said he would have preferred the vaccines had been made available under what’s known as an expanded access protocol — basically, a big clinical trial — and not an EUA.
https://www.statnews.com/2020/12/17/moderna-vaccine-fda-panel/
Doc Robinson
ParticipantSeems like the FDA can deflect blame by having an external advisory committee vote in favor of the mRNA injections. Interesting that the FDA clarifies that the Pfizer vaccine is still “not an approved product” despite the EUA and the massive push to vaccinate the public. The consent form for Pfizer and Moderna’s vaccines should make it clear that these vaccines are still not approved by the FDA.
From the FDA’s document to brief the advisory committee about the Moderna vaccine:
2.4 Alternatives for Prevention of COVID-19No vaccine or other medical product is FDA approved for prevention of COVID-19. On December 11, 2020, FDA issued an EUA for the Pfizer-BioNTech COVID-19 vaccine for active immunization for prevention of COVID-19 due to SARS-CoV-2 in individuals 16 years of age and older. However, the Pfizer-BioNTech COVID-19 vaccine is not an approved product, and furthermore is not available in quantity sufficient to vaccinate all persons in the U.S. for whom the vaccine is authorized for use. On October 22, 2020, FDA approved remdesivir for use in adult and pediatric patients 12 years of age and older and weighing at least 40 kilograms for the treatment of COVID-19 requiring hospitalization. Several other therapies are currently available under emergency use authorization, but not FDA approved, for treatment of COVID-19. Thus, there is currently no adequate, approved, and available alternative for prevention of COVID-19.
https://www.fda.gov/media/144434/download
The FDA did approve remdesivir for treatment of hospitalized Covid-19 patients, despite the WHO trial showing lack of efficacy. So they approve a treatment that doesn’t work, and they withhold approval for treatments that do work (like ivermectin), and push the widespread usage of these vaccines without actually approving the vaccines.
Covid-19: US approves remdesivir despite WHO trial showing lack of efficacy
https://www.bmj.com/content/371/bmj.m4120Doc Robinson
Participant@ teri, thank you for that summary.
The listing is for the Moderna product, and the Pfizer product is similar (but I haven’t parsed the details for any differences between the two).
Doc Robinson
ParticipantThe Moderna vaccine is on track to get an Emergency Use Authorization (EAU) from the FDA tomorrow. The advisory committee meets today.
An expert panel is meeting Thursday to consider whether the Food and Drug Administration should issue a second emergency use authorization for a Covid-19 vaccine, this one made by Moderna.
It is almost a foregone conclusion that it will. But the hearing still promises to tell us more about the vaccine and its use.
The FDA gave Moderna’s vaccine a favorable review in the leadup to the meeting, all but guaranteeing the Vaccines and Related Biological Products Advisory Committee will recommend an EUA be granted. It’s also widely expected the FDA will issue the EUA on Friday.
https://www.statnews.com/2020/12/17/moderna-vaccine-fda-panel/
In the briefing document for that meeting, the many unknowns and risks of the Moderna vaccine are summarized. Some snippets from the document:
8.2 Unknown Benefits/Data GapsDuration of protection
…it is not possible to assess sustained efficacy over a period longer than 2 months.Effectiveness in certain populations at high-risk of severe COVID-19
…subsets of certain groups such as immunocompromised individuals (e.g., those with HIV/AIDS)…Effectiveness in individuals previously infected with SARS-CoV-2
…participants with a known history of SARSCoV-2 infection were excluded from the Phase 3 study…Effectiveness in pediatric populations
No efficacy data are available from participants ages 17 years and younger.Future vaccine effectiveness as influenced by characteristics of the pandemic, changes in the virus, and/or potential effects of co-infections
…The evolution of the pandemic characteristics, such as increased attack rates, increased exposure of subpopulations, as well as potential changes in the virus infectivity, antigenically significant mutations to the S protein, and/or the effect of coinfections may potentially limit the generalizability of the efficacy conclusions over time…Vaccine effectiveness against asymptomatic infection
Vaccine effectiveness against long-term effects of COVID-19 disease
Vaccine effectiveness against mortality
Vaccine effectiveness against transmission of SARS-CoV-2
8.3 Known Risks
The vaccine elicited increased local and systemic adverse reactions as compared to those in the placebo arm, usually lasting a few days. The most common solicited adverse reactions were pain at injection site (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%). Adverse reactions characterized as reactogenicity were generally mild to moderate; 0.2% to 9.7% of these events were reported as severe, with severe solicited adverse reactions being more frequent after dose 2 than after dose 1 and generally less frequent in older adults (≥65 years of age) as compared to younger participants. Among reported unsolicited adverse events, lymphadenopathy occurred much more frequently in the vaccine group than the placebo group and is plausibly related to vaccination. The number of participants reporting hypersensitivity-related adverse events was numerically higher in the vaccine group compared with the placebo group (258 events in 233 participants [1.5%] vs. 185 events in 166 participants [1.1%]). There were no anaphylactic or severe hypersensitivity reactions with close temporal relation to the vaccine. Serious adverse events, while uncommon (1.0% in both treatment groups), represented medical events that occur in the general population at similar frequency as observed in the study. Of the 7 SAEs in the mRNA-1273 group that were considered as related by the investigator, FDA considered 3 as related: intractable nausea and vomiting (n=1), facial swelling (n=2). For the serious adverse events of rheumatoid arthritis, peripheral edema/dyspnea with exertion, and autonomic dysfunction, a possibility of vaccine contribution cannot be excluded. For the event of B-cell lymphoma, an alternative etiology is more likely. An SAE of Bell’s palsy occurred in a vaccine recipient, for which a causal relationship to vaccination cannot be concluded at this time. No specific safety concerns were identified in subgroup analyses by age, race, ethnicity, medical comorbidities, or prior SARS-CoV-2 infection.
8.4 Unknown Risks/Data Gaps
Safety in certain subpopulations
…such as children less than 18 years of age, pregnant and lactating individuals, and immunocompromised individuals.Adverse reactions that are very uncommon or that require longer follow-up to be detected
…use in large numbers of individuals may reveal additional, potentially less frequent and/or more serious adverse events not detected in the trial..Vaccine-enhanced disease
…risk of vaccine-enhanced disease over time, potentially associated with waning immunity, remains unknown and needs to be evaluated further in ongoing clinical trials and in observational studies that could be conducted following authorization and/or licensure.Vaccines and Related Biological Products Advisory Committee Meeting
December 17, 2020
FDA Briefing Document
Moderna COVID-19 Vaccine
https://www.fda.gov/media/144434/downloadDoc Robinson
ParticipantI previously mentioned the presence of Polyethylene Glycol (PEG) in the Pfizer vaccine. And unless Moderna states otherwise, there’s a good chance it’s a component of their vaccine, too. It’s used with the lipid nanoparticles coating the mRNA.
“BioNTech/Pfizer and Moderna encapsulate their mRNA vaccines within LNPs [lipid nanoparticles].”
https://pubs.acs.org/doi/10.1021/acsnano.0c07197#An unknown percentage of the population will have reactions to PEG being injected into their bodies.
“Polyethylene glycol (PEG)-coated nanopharmaceuticals can cause mild to severe hypersensitivity reactions (HSRs), which can occasionally be life threatening or even lethal. The phenomenon represents an unsolved immune barrier to the use of these drugs, yet its mechanism is poorly understood…”
https://pubs.acs.org/doi/10.1021/acsnano.9b03942It might be happening already in the US with the Pfizer vaccine, to people without any known allergies:
A health worker in Alaska experienced a serious allergic reaction just 10 minutes after receiving Pfizer’s coronavirus vaccine on Tuesday, marking the first adverse effect from the vaccine reported in the U.S.The health worker experienced an anaphylactic reaction and was taken to the emergency room, then later transferred to the intensive care unit, Alaska state health officials said.
The patient did not have a previous history of drug related allergies, the officials confirmed during a call with reporters. She experienced shortness of breath, elevated heart rate and a rash on her face and torso. She was treated with epinephrine and antihistamines, but her symptoms recurred multiple times and she was taken to the ICU. Officials said by Wednesday she had recovered but remains in the ICU for monitoring.
“This is the only case in the U.S., but this doesn’t mean there won’t be more cases,” said Dr. Jay Butler, deputy director for Infectious Diseases at the CDC.
https://www.politico.com/news/2020/12/16/alaska-pfizer-coronavirus-vaccine-reaction-446861
Doc Robinson
ParticipantThe UK government disclosed the ingredients of the Pfizer vaccine:
What [Pfizer] COVID-19 mRNA Vaccine BNT162b2 contains
• The active substance is BNT162b2 RNA.
After dilution, the vial contains 5 doses, of 0.3 mL with 30 micrograms mRNA each.
• This vaccine contains polyethylene glycol/macrogol (PEG) as part of ALC-0159
• The other ingredients are:
– ALC-0315 = (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate),
– ALC-0159 = 2[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide,
– 1,2-Distearoyl-sn-glycero-3-phosphocholine, 5
– cholesterol,
– potassium chloride,
– potassium dihydrogen phosphate,
– sodium chloride,
– disodium hydrogen phosphate dihydrate,
– sucrose
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/943249/Information_for_UK_recipients.pdfOfficial consent form:
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/942009/PHE_11843_Covid-19_Consent_form_adults_able_to_consent.pdfConsent form suggested by UK Medical Freedom Alliance:
https://uploads-ssl.webflow.com/5fa5866942937a4d73918723/5fc76c8cbd4d75f7ea592501_UKMFA_CV19_vaccine_consent_form_v1-1.pdfDoc Robinson
ParticipantPennsylvania Republicans Ask SCOTUS To Again Review Election Lawsuit
In addition to the Pennsylvania lawsuit, two of Sidney Powell’s lawsuits (Michigan and Georgia) were put on the SCOTUS docket yesterday.
Powell is seeking to challenge those rulings directly at the Supreme Court, bypassing the federal appeals court level.The cases are King v. Whitmer, 20-815, and In Re Pearson, 20-816.
https://www.supremecourt.gov/search.aspx?filename=/docket/docketfiles/html/public/20-815.html
https://www.supremecourt.gov/search.aspx?filename=/docket/docketfiles/html/public/20-816.htmlDoc Robinson
ParticipantFrom that market-ticker article linked above:
“Because under the law an EUA can only be issued if there are no safe and effective treatments. If there are safe and effective treatments [like Ivermectin] then under the law an EUA cannot issue; you must instead go through the entire procedure to get regular approval.”
Like Huskynut said, if you ain’t angry, you don’t understand what’s going down. The official briefing documents linked below (for the advisory committee that voted in favor of approving the Pfizer vaccine) have no mention of Ivermectin, and the claim that there is currently “no broadly effective treatment or prevention available is used to justify the approval of the vaccine.”
8. RISK/BENEFIT ASSESSMENT
The ongoing COVID-19 pandemic has a significant impact on public health, and currently there is no broadly effective treatment or prevention available. An effective vaccine can impact the pandemic at this critical time. According to the Institute for Health Metrics and Evaluation, there will be >55,000 deaths per month in the US over the next few months.25 A COVID-19 vaccination program implemented soon can likely prevent many deaths.26 A vaccine must be introduced before the peak of reported cases to have a significant impact on the pandemic course.26,27 A highly effective vaccine, with sufficient uptake as supplies become available, may be able to induce population herd immunity to bring the pandemic under control.
Doc Robinson
ParticipantI guess I should have written “…according to the CDC (for what it’s worth).”
Somebody used the CDC data for 2015-2018 total death numbers here, but they reportedly used misleading numbers for 2019 and 2020:

https://static.politifact.com/politifact/photos/US_Death_toll_meme.jpgFirstly, the numbers listed from 2015 through 2018 are official, having been released by the CDC ( here , here , here , here ). However, the final numbers for 2019 have not yet been released, and it is unclear where the image creator took the 2,900,689 figure from. This is the same for the 2020 projection.
Meanwhile, the 2020 figure leading up to Nov. 16 is also an official figure released by the CDC (pdf here ) – but it is not yet an accurate representation of how many people have died in 2020. The table doesn’t begin until the week ending Feb. 1, 2020 and still has six weeks to report between Nov. 16 and the end of the year. The latest weekly data in the Nov. 16 chart also appears to have a much lower death count than previous weeks, which the CDC has said is due to a lag in reporting. This lag can be anywhere between one and eight weeks, or more...
https://www.reuters.com/article/uk-factcheck-chart-us-death-figures-2020-idUSKBN2872MV
The CDC data is not very user-friendly in my experience, and more than once I’ve thought “This doesn’t add up.”
Dr. D: “I JUST WANT THE NUMBER OF PEOPLE WHO DIED GOING BACK TO 2010.”
You’d think the CDC would have this type of overview easily accessible, but the closest I found was a summary from USA Today (based on CDC data):
2010
• Age-adjusted death rate: 747 deaths per 100,000 people
• Avg. life expectancy: 78.7 years
• Deaths: 2,468,435
• Population: 309,346,863
• Leading cause of death: Heart disease (597,689 deaths, 24.2% of all deaths)
• Second leading cause of death: Cancer (574,743 deaths, 23.3% of all deaths)
• Third leading cause of death: Chronic lower respiratory diseases (138,080 deaths, 5.6% of all deaths)2011
• Age-adjusted death rate: 741 deaths per 100,000 people
• Avg. life expectancy: 78.7 years
• Deaths: 2,515,458
• Population: 311,718,857
• Leading cause of death: Heart disease (596,577 deaths, 23.7% of all deaths)
• Second leading cause of death: Cancer (576,691 deaths, 22.9% of all deaths)
• Third leading cause of death: Chronic lower respiratory diseases (142,943 deaths, 5.7% of all deaths)2012
• Age-adjusted death rate: 733 deaths per 100,000 people
• Avg. life expectancy: 78.8 years
• Deaths: 2,543,279
• Population: 314,102,623
• Leading cause of death: Heart disease (599,711 deaths, 23.6% of all deaths)
• Second leading cause of death: Cancer (582,623 deaths, 22.9% of all deaths)
• Third leading cause of death: Chronic lower respiratory diseases (143,489 deaths, 5.6% of all deaths)2013
• Age-adjusted death rate: 732 deaths per 100,000 people
• Avg. life expectancy: 78.8 years
• Deaths: 2,596,993
• Population: 316,427,395
• Leading cause of death: Heart disease (611,105 deaths, 23.5% of all deaths)
• Second leading cause of death: Cancer (584,881 deaths, 22.5% of all deaths)
• Third leading cause of death: Chronic lower respiratory diseases (149,205 deaths, 5.7% of all deaths)2014
• Age-adjusted death rate: 725 deaths per 100,000 people
• Avg. life expectancy: 78.9 years
• Deaths: 2,626,418
• Population: 318,907,401
• Leading cause of death: Heart disease (614,348 deaths, 23.4% of all deaths)
• Second leading cause of death: Cancer (591,700 deaths, 22.5% of all deaths)
• Third leading cause of death: Chronic lower respiratory diseases (147,101 deaths, 5.6% of all deaths)2015
• Age-adjusted death rate: 733 deaths per 100,000 people
• Avg. life expectancy: 78.7 years
• Deaths: 2,712,630
• Population: 321,418,820
• Leading cause of death: Heart disease (633,842 deaths, 23.4% of all deaths)
• Second leading cause of death: Cancer (595,930 deaths, 22.0% of all deaths)
• Third leading cause of death: Chronic lower respiratory diseases (155,041 deaths, 5.7% of all deaths)2016
• Age-adjusted death rate: 729 deaths per 100,000 people
• Avg. life expectancy: 78.7 years
• Deaths: 2,744,248
• Population: 323,071,342
• Leading cause of death: Heart disease (635,260 deaths, 23.1% of all deaths)
• Second leading cause of death: Cancer (598,038 deaths, 21.8% of all deaths)
• Third leading cause of death: Accidents (unintentional injuries) (161,374 deaths, 5.9% of all deaths)2017
• Age-adjusted death rate: 732 deaths per 100,000 people
• Avg. life expectancy: 78.6 years
• Deaths: 2,813,503
• Population: 325,147,121
• Leading cause of death: Heart disease (647,457 deaths, 23.0% of all deaths)
• Second leading cause of death: Cancer (599,108 deaths, 21.3% of all deaths)
• Third leading cause of death: Accidents (unintentional injuries) (169,936 deaths, 6.0% of all deaths)2018
• Age-adjusted death rate: 724 deaths per 100,000 people
• Avg. life expectancy: 78.7 years
• Deaths: 2,839,205
• Population: 327,167,439
• Leading cause of death: Heart disease (655,381 deaths, 23.1% of all deaths)
• Second leading cause of death: Cancer (599,274 deaths, 21.1% of all deaths)
• Third leading cause of death: Accidents (unintentional injuries) (167,127 deaths, 5.9% of all deaths)Doc Robinson
Participant@ madamski, I believe that BS pronouncements tend to erode credibility, despite the truths also being presented. Like shooting oneself in the foot.
Still, if someone is against “fake news” and propaganda, then proclaims something like “no additional deaths at all,” I tend to give them the benefit of the doubt and assume they are misinformed and not hypocritical.
Doc Robinson
ParticipantDr. D: “So weird how we have these mass pandemics with NO ADDITIONAL DEATHS at all.”
That’s quite a claim. In the US, the CDC has been counting the number of deaths from all causes, and total deaths in 2020 are roughly 10% more than previous years.
I like this multi-year graph format because the y-axis goes all the way down to zero, allowing for any increases to be put in perspective relative to the total.
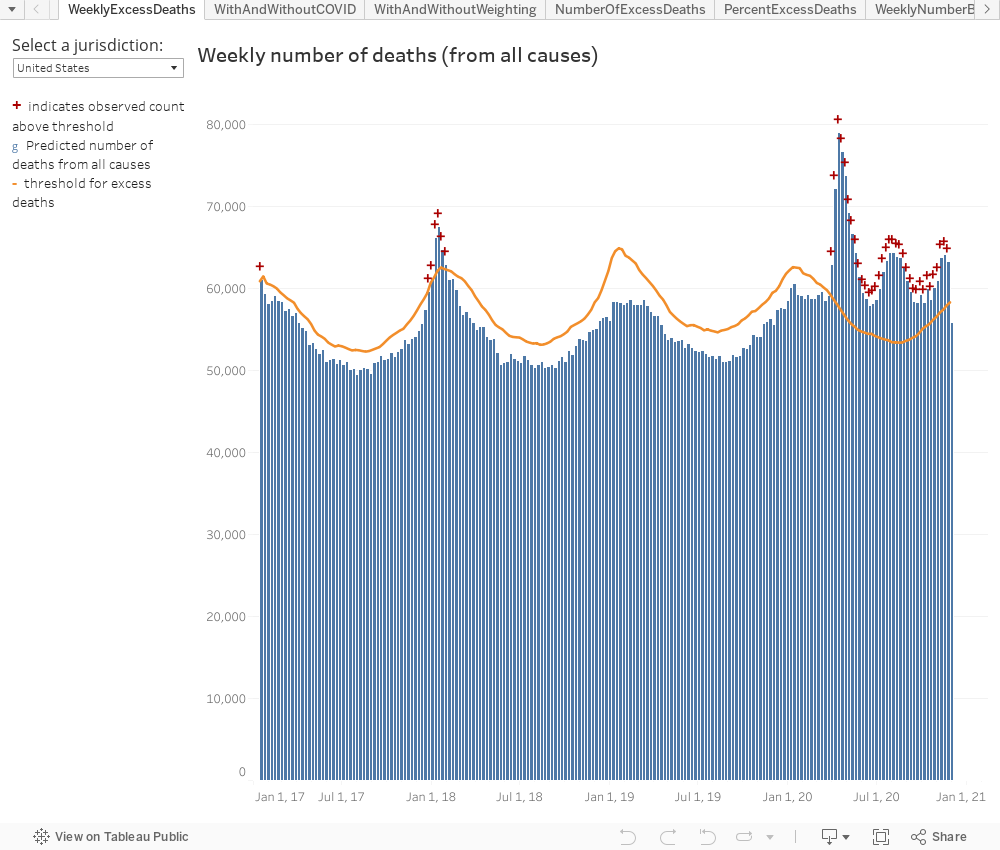
https://www.cdc.gov/nchs/nvss/vsrr/covid19/excess_deaths.htmNumber of deaths reported on this page are the total number of deaths received and coded as of the date of analysis and do not represent all deaths that occurred in that period. Data are incomplete because of the lag in time between when the death occurred and when the death certificate is completed, submitted to NCHS and processed for reporting purposes. This delay can range from 1 week to 8 weeks or more, depending on the jurisdiction and cause of death…
Finally, the estimates of excess deaths reported here may not be due to COVID-19, either directly or indirectly. The pandemic may have changed mortality patterns for other causes of death. Upward trends in other causes of death (e.g., suicide, drug overdose, heart disease) may contribute to excess deaths in some jurisdictions.
Doc Robinson
Participant‘Like Nothing Happened’: Sydney Restaurants Are Bustling (AFR)
Now Australia is at the point where they are just locking down their borders, instead of locking down their people. Back in July it was looking grim there.
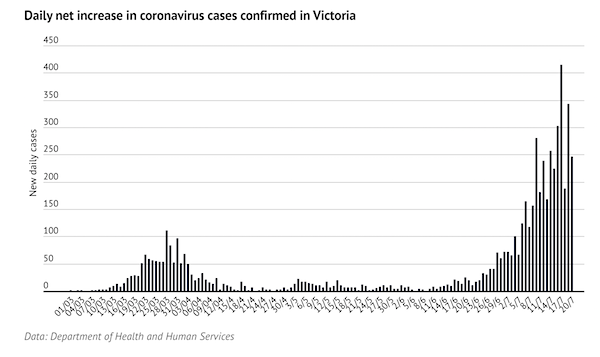
Looking back at the Debt Rattle for July 22 2020:
It Has Become Clear Elimination Of The Virus Is The Best Option (Age)
…Our already-stretched health system is at risk of being stretched beyond its limit. We have tried to suppress this virus; to keep infection numbers low enough to allow our hospitals to cope, while not actively trying to stamp it out. In Melbourne, it has failed… Many have argued for an explicit elimination strategy, acknowledging that while not without its costs, ultimately it is the best choice for our society and economy…Yet the current six-week lockdown is unlikely to eliminate the virus, according to new analysis in the Medical Journal of Australia. We must either go harder, or go longer.
While it is the best option for Australia, elimination has three important drawbacks: its impacts would be disproportionately felt by disadvantaged and marginalised people; it would require a longer, more intense period of initial lockdown; and it would make us dependent on a vaccine… Better to do it once (or twice, as in Melbourne) and do it properly, rather than wait for infections to rise again in a few months and have to do it all again.
If we succeed in ridding ourselves of SARS-CoV-2, we will make ourselves reliant on a vaccine. Until one is found, our borders will have to remain closed. The virus will become endemic in many other countries – countries who, unlike us, no longer have the option of eliminating it. Opening our borders too early would simply be kicking the can down the road.
https://www.theautomaticearth.com/2020/07/debt-rattle-july-22-2020/
Doc Robinson
Participant“How many Australians are still stuck abroad after 11 months?”
More than 30,000 are reportedly waiting to return to Australia.
In January, about 2.3 million people came to Australia. By September, the figure was 16,720…A similar collapse in travel has occurred across Asia, where restrictions are tougher than in the United States and Europe, though without Australia’s tight quarantine caps. Visitors to Japan fell to 13,700 in September from 2.7 million in January; to 64,000 from 1.3 million in South Korea over the same period; and to 13,800 from 2 million in Vietnam…
It is the quarantine system that has allowed us to go back to a semi-normal state… When they arrive, travelers are escorted under police or military guard to sealed hotels, where they are not allowed to leave their rooms…
The last coronavirus case diagnosed in the community in Australia was a cleaner, on Dec. 3, who works in a hotel where travelers from overseas are locked in their rooms. Among those in quarantine, 62 cases were identified over the past week — cases that policymakers say illustrate the danger of repatriating more citizens and residents.
Doc Robinson
ParticipantThe “Chief Strategy Officer” for the Bill & Melinda Gates Foundation is an economist.
Leadership
Bill Gates, Co-chair and Trustee
Melinda Gates, Co-chair and Trustee
Warren Buffett, Trustee
William H. Gates Sr., Former Co-chair (d. 2020)
Mark Suzman, Chief Executive Officer
Carolyn Ainslie, Chief Financial Officer
Lisa Alvarez-Calderón, Chief Human Resources Officer
Susan Byrnes, Chief Communications Officer
Connie Collingsworth, Chief Operating Officer
Chris Elias, President, Global Development
Gargee Ghosh, President, Global Policy & Advocacy
Allan C. Golston, President, United States Program
Trevor Mundel, President, Global Health
Rodger Voorhies, President, Global Growth & Opportunity
Ankur Vora, Chief Strategy Officer
Global Development Division Leadership
Global Growth & Opportunity Division Leadership
Global Health Division Leadership
Global Policy & Advocacy Division Leadership
United States Program Leadership
Operations Division Leadershiphttps://www.gatesfoundation.org/Who-We-Are/General-Information/Foundation-Factsheet
Doc Robinson
ParticipantTexas replied to the Supreme Court today. A summary of their arguments (from the Table of Contents):
Argument………………………………………………………….. 3
I. Texas IS likely to prevail………………………………. 3
A. Defendant States violated the Electors
Clause by modifying their legislatures’
election laws through non-legislative
action…………………………………………………….. 3B. State and local administrator’s systemic
failure to follow State election law
qualifies as an unlawful amendment of
State law. ………………………………………………. 5C. Defendant States’ invocation of other
litigation does not affect this action,
either substantively or jurisdictionally. ……. 5D. Texas has standing to sue……………………….. 7
E. Neither laches nor mootness bar
injunctive relief………………………………………. 9II. The other Winter-Hollingsworth factors
warrant interim relief…………………………………. 10A. Plaintiff State will suffer irreparable
harm if the Defendant States’
unconstitutional presidential electors
vote in the Electoral College. …………………. 10B. The balance of equities tips to the
Plaintiff State. ……………………………………… 10C. The public interest favors interim relief. … 11
Conclusion ………………………………………………………. 12
Doc Robinson
ParticipantDr. D: Yes, literally in the United States if you come from Spain, you are “hispanic” not “White”. You are “Ethnic” not “European.” Explain?
Not quite. From the latest results of the US Census (2010):
“People who identify their origin as Hispanic, Latino, or Spanish may be any race.”
“Hispanic or Latino” refers to a person of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin regardless of race.
“White” refers to a person having origins in any of the original peoples of Europe, the Middle East, or North Africa. It includes people who indicated their race(s) as “White” or reported entries such as Irish, German, Italian, Lebanese, Arab, Moroccan, or Caucasian.
“Over half of the Hispanic population identified as White and no other race…”
Doc Robinson
ParticipantWES — If 17 out of 22 experts think your bridge is safe-ish, then I want to know why the other experts don’t agree with them.
Doc Robinson
ParticipantFDA Panel Recommends COVID-19 Vaccine For Emergency Use
In a 17-4 vote, with one abstention, a panel of advisers to the Food and Drug Administration recommended Thursday that the COVID-19 vaccine being developed by Pfizer and BioNTech be authorized for emergency use during the pandemic.
The vote in favor of the vaccine was taken to answer the agency’s question: Do the benefits of the Pfizer-BioNTech COVID-19 vaccine outweigh its risks for use in people age 16 and older..
17 out of 22 “expert advisors” voted that the vaccine’s benefits outweigh the risks. Why would the other experts on the panel not join in recommending the vaccine? Perhaps it has something to do with the Unknown Benefits, Known Risks, Unknown Risks, and Data Gaps associated with the vaccine (conveniently summarized in this committee meeting briefing document sponsored by Pfizer and BioNTech).
8.2. Unknown Benefits/Data Gaps
Duration of protection
Effectiveness in certain populations at high-risk of severe COVID-19
Effectiveness in individuals previously infected with SARS-CoV-2
Effectiveness in pediatric populations
Future vaccine effectiveness as influenced by characteristics of the pandemic, changes in the virus, and/or potential effects of co-infections
Vaccine effectiveness against asymptomatic infection
Vaccine effectiveness against long-term effects of COVID-19 disease
Vaccine effectiveness against mortality
Vaccine effectiveness against transmission of SARS-CoV-28.3. Known Risks
…The most common solicited adverse reactions were injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%).
…Severe adverse reactions occurred in 0.0-4.6% of participants, were more frequent after Dose 2…8.4. Unknown Risks/Data Gaps
Safety in certain subpopulations
Adverse reactions that are very uncommon or that require longer follow-up to be detected
Vaccine-enhanced disease
…risk of vaccine-enhanced disease over time, potentially associated with waning immunity, remains unknown and needs to be evaluated further in ongoing clinical trials…Doc Robinson
ParticipantLots of activity today on the Supreme Court docket for Texas v. Pennsylvania et al., including Washington DC filing on behalf of itself and 22 states and territories in support of the defendents. Lots of people getting involved (on both sides).
Dec 10 2020 Motion of State of Ohio for leave to file amicus brief not accepted for filing. (December 10, 2020) (corrected motion electronically filed)
Dec 10 2020 Response to motion for leave to file bill of complaint and motion for preliminary injunction and temporary restraining order or stay from defendant Wisconsin filed.
Dec 10 2020 Opposition to Texas’s motion for leave to file bill of complaint and its motion for preliminary relief from defendant Georgia filed.
Dec 10 2020 Opposition to motions for leave to file bill of complaint and for injunctive relief from defendant Michigan filed.
Dec 10 2020 Opposition to motion for leave to file bill of complaint and motion for preliminary injunction, temporary restraining order, or stay from defendant Pennsylvania filed.
Dec 10 2020 Motion for leave to file amicus brief from the District of Columbia on behalf of 22 States and Territories filed.
Dec 10 2020 Motion to Intervene and Proposed Bill of Complaint in Intervention of State of Missouri submitted.
Dec 10 2020 Motion of State of Ohio for leave to file amicus brief submitted.
Dec 10 2020 Motion for Leave to File Brief as Amicus Curiae and Brief for Members of the Pennsylvania General Assembly. as Amicus Curiae in Support of Plaintiff/Defendants of Members of the Pennsylvania General Assembly submitted.
Dec 10 2020 Motion of Certain Select Pennsylvania State Senators for leave to file amicus brief submitted.
Dec 10 2020 Motion of Christian Family Coalition for leave to file amicus brief submitted.
Dec 10 2020 Amicus brief of Bryan Cutler Speaker of the Pennsylvania House of Representatives and Kerry Benninghoff Majority Leader of the Pennsylvania House of Representatives submitted.
Dec 10 2020 For Leave to File Complaint-in-Intervention of Ron Heuer, et al. submitted.
Dec 10 2020 Motion of U.S. Representative Mike Johnson and 105 Other Members for leave to file amicus brief submitted.
Dec 10 2020 Motion of Lieutenant Governor Janice McGeachin, Senator Lora Reinbold, Representative David Eastment, et al. (Elected State Officials) for leave to file amicus brief submitted.
Dec 10 2020 Amicus brief of City of Detroit submitted.
Dec 10 2020 Motion for Leave to File and Amicus Curiae Brief of Justice and Freedom Fund in Support of Plaintiff of Justice and Freedom Fund in Support of Plaintiff submitted.https://www.supremecourt.gov/docket/docketfiles/html/public/22o155.html
Doc Robinson
Participant@ sumac.carol
Thanks for the additional context. Without going back to re-read the article, my comment was in response to your summary about simpler protein profiles: “Jon argues that the covid vaccine is less likely than the regular flu shot to generate adverse reactions because the covid vaccines have much simpler protein profiles.”
In any case, the Pfizer trials don’t seem to support the claim that “the covid vaccine is less likely than the regular flu shot to generate adverse reactions.”
Doc Robinson
ParticipantSome quotations from the Jon Barron article linked above:
“In any case, at the moment, until the vaccine is used at much higher levels and we get to see if there are any long-term complications, the safety of these vaccines is an open-ended question…
“And remember, even those who have been vaccinated will silently lose their protection, at any point in time, and with no easy way to know when that had happened.”
“That means that annual mass vaccination for COVID will be a likely reality for at least several years to come.”
Doc Robinson
Participantsumac.carol: “In the link above to Jon Barron’s article, Jon argues that the covid vaccine is less likely than the regular flu shot to generate adverse reactions because the covid vaccines have much simpler protein profiles.”
I read Jon Barron’s article when the link was posted earlier (thanks carol), and that claim –less adverse reactions because of simpler protein profiles– stuck out because it’s not supported by the Pfizer trials, in which the simpler protein gave worse side effects.
Pfizer initially tested two vaccines, BNT162b1 and BNT162b2, abbreviated as b1 and b2.
“Both use messenger RNA to instruct cells to make viral proteins in an attempt to stimulate an immune response; the difference is that cells receiving b1 make part of the SARS-CoV-2 virus’ “spike” protein, while b2 stimulates production of the entire protein.”
b2 (with the entire protein) had less side effects than b1 (with only part of the protein), and b2 was chosen because of this.
In the case of b1, three-quarters of patients aged 18 to 55 and one-third of patients aged 65 to 85 experienced fever after the second of two shots of either 10 or 30 micrograms. For b2 at 30 micrograms, 17% of patients 18 to 55 and 8% of those 65 to 85 reported fever after the second dose.
By comparison, Phase 1 results showed Moderna’s vaccine mRNA-1273, which also uses mRNA, led to fever in 40% of patients injected with the 100-microgram dose selected for Phase 3 trials.
https://www.biopharmadive.com/news/pfizer-biontech-coronavirus-vaccine-choice-safety/583878/
Doc Robinson
ParticipantProblems on the first day of the UK’s vaccination program:
“British regulators warned Wednesday that people who have a history of serious allergic reactions shouldn’t receive the new Pfizer-BioNTech vaccine as they investigate two adverse reactions that occurred on the first day of the country’s mass vaccination program.”
“The medical regulatory agency also said vaccinations should not be carried out in facilities that don’t have resuscitation equipment.”
“Documents published by the two companies showed that people with a history of severe allergic reactions were excluded from the trials, and doctors were advised to look out for such reactions in trial participants who weren’t previously known to have severe allergies.”
“The U.K. began its mass vaccination program on Tuesday, offering the shot to people over 80, nursing home staff and some NHS workers. It’s not clear how many people have received the jab so far.”
https://www.ctvnews.ca/world/u-k-probing-if-allergic-reactions-linked-to-pfizer-vaccine-1.5223107
Doc Robinson
Participant“…in the Pfizer vaccine group two volunteers just died.”
The linked JP article gives some additional context. Two died in the vaccine group, while four died in the placebo group.
“According to the published data, six of the participants in the experiment died, two of whom received the vaccine and four of the control group,” said Dr. Uri Lerner, the scientific director for Midaat. “After an in-depth examination, no connection was found between the experiment and the cause of death.”
Doc Robinson
ParticipantJohn Day (in yesterday’s comments): “What is the relative risk between each vaccinated group and each placebo control group, of feeling bad enough to stay home in bed for one or more days?”
Data such as this seems to be withheld from public knowledge. The media relies on the limited information that the drug companies put in their press releases. For example, the Pfizer press release disclosed these numbers about side effects:
“The only Grade 3 (severe) solicited adverse events greater than or equal to 2% in frequency after the first or second dose was fatigue at 3.8% and headache at 2.0% following dose 2.”
This leaves out any “severe” reactions reported by less than 2% of participants.
This also leaves out all of the “moderate” reactions.I had to look back to some Pfizer trial data published in August to find what is considered to be a “moderate” and “severe” reaction:
A fever of 38.9°C (102°) qualifies as a “moderate” fever.
Headaches and fatigue and muscle pain are considered “moderate” if they cause interference with activity (to qualify as “severe” it would have to prevent daily activity).So this Pfizer data indicates that an undisclosed percentage of their vaccine recipients had side effects bad enough to cause interference with activity, and 3.8%,of recipients got fatigue that prevented daily activity, and 2% of the recipients got headaches that prevented daily activity. (These are just the known side effects in the short term.)
Once the public vaccinations begin, I expect there will be significant numbers of people who are unhappy about their bad experiences with the side effects. Word-of-mouth stories from family and friends will be more persuasive than a press release saying that the vaccine is “well tolerated across all populations.”
It seems that a media campaign to downplay side effects has already begun. Some recent headlines:
USA TODAY
Side effects from the COVID-19 vaccine means ‘your body responded the way it’s supposed to,’ experts sayWashington Post
‘Absolutely normal’: Covid vaccine side effects are no reason to avoid the shots, doctors sayThe Philadelphia Inquirer
COVID-19 vaccines can cause side effects. Here’s why that shouldn’t stop you from getting the shots.Science
Public needs to prep for vaccine side effectsCNBC
Trump Covid vaccine czar says side effects ‘significantly noticeable’ in [only] 10% to 15% of recipientsPfizer press release
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccineearlier Pfizer vaccine trial data published in August
https://www.nature.com/articles/s41586-020-2639-4/figures/3Doc Robinson
Participantchettt: “How might the human challenge be done? Will they just put volunteers in a room with infected people?”
How an earlier trial for the flu was done: “…he developed a challenge model using a wildtype strain of H1N1 that has since been sprayed up the noses of more than 400 volunteers.”
Doc Robinson
Participant@ chettt, comparing to the effectiveness of the measles vaccine:
“…So to prevent one severe illness 1370 individuals must be vaccinated. The other 1369 individuals are not saved from a severe illness, but are subject to vaccine adverse effects, whatever they may be and whenever we learn about them. How does this compare with other vaccines? Before the measles vaccine became available 90% of children in North America had measles by age 10. Two doses of the vaccine are about 95% effective, so a vaccinated individual’s risk is reduced by 0.855 (0.90 x 0.95), and the NNTV [Number Needed To Vaccinate] = 1.17 (1/0.855); this is extraordinarily effective…Shouldn’t absolute risk reduction be reported so individuals can make fully informed decisions about vaccinations?”
Doc Robinson
Participantcfraenkel’s arguments contain false equivalences and guilt by association fallacies.
I highlighted the concerns of Peter Doshi (an editor at the BMJ) and others published by that journal. Effectiveness is just one of the multiple concerns they raise.
Regarding the use of relative or absolute risk reduction numbers, here’s some advice published online by the National Institute of Health:
“Absolute risk reduction (ARR) – also called risk difference (RD) – is the most useful way of presenting research results to help your decision-making.”
Doc Robinson
ParticipantupstateNYer: “..in what manner were these two groups of guinea pigs, um, I mean people, exposed to the virus? How was it determined that exposure was equal for both groups?”
As far as I know, there was no intentional exposure, but in a randomized study both groups should ideally have similar demographics and similar enough exposure during their daily lives.
Intentionally infecting the subjects is planned in some upcoming “human challenge” trials.
Young, healthy people will be intentionally exposed to the virus responsible for COVID-19 in a first-of-its kind ‘human challenge trial’, the UK government and a company that runs such studies announced on 20 October. The experiment, set to begin in January in a London hospital if it receives final regulatory and ethical approval, aims to accelerate the development of vaccines that could end the pandemic.
Human challenge trials have a history of providing insight into diseases such as malaria and influenza. The UK trial will try to identify a suitable dose of the virus SARS-CoV-2 that could be used in future vaccine trials. But the prospect of deliberately infecting people — even those at low risk of severe disease — with SARS-CoV-2, a deadly pathogen that has few proven treatments, is uncharted medical and bioethical territory.
Dozens to be deliberately infected with coronavirus in UK ‘human challenge’ trials
https://www.nature.com/articles/d41586-020-02821-4 -
AuthorPosts